Overview
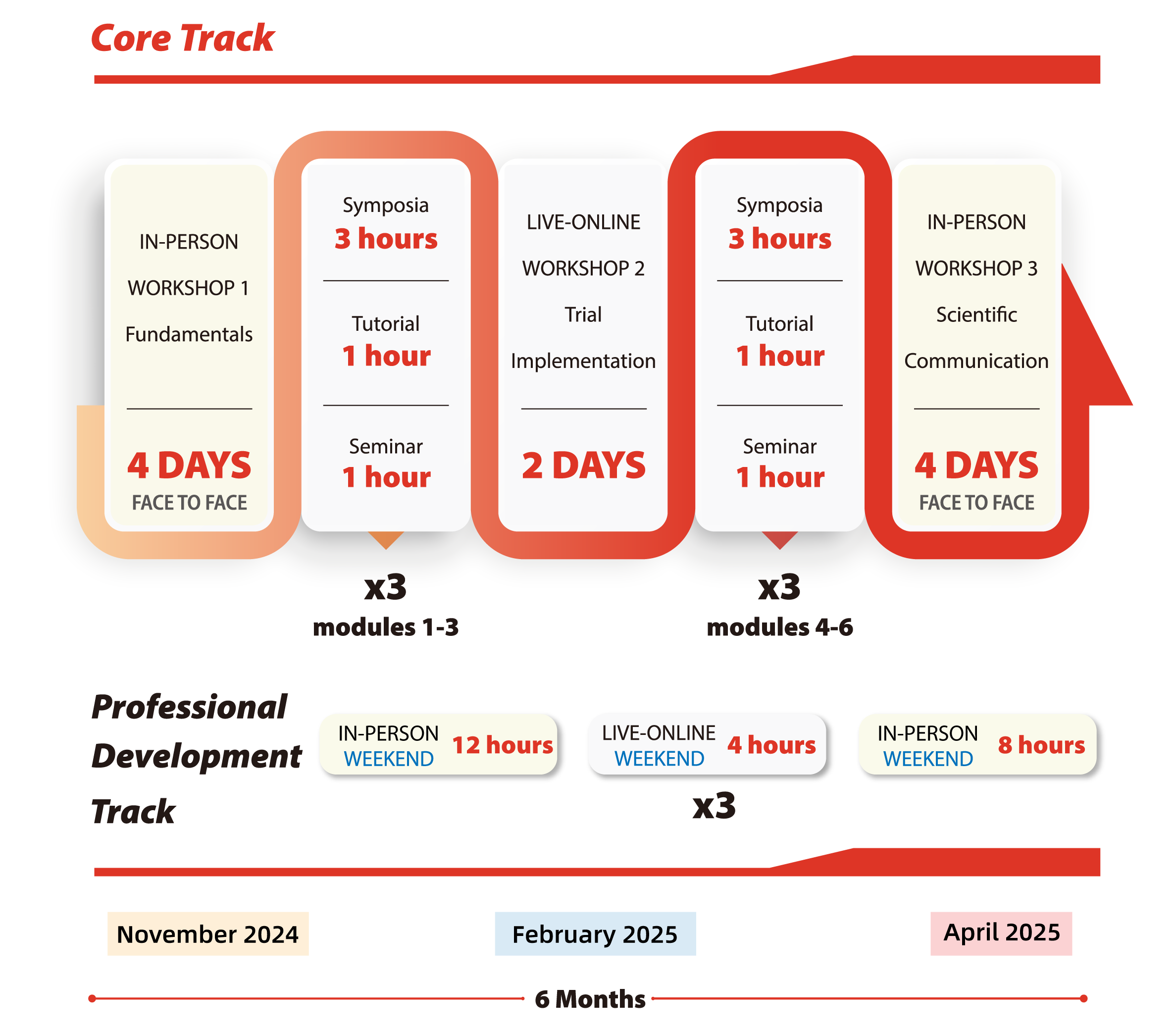
NEJM高水平临床研究培训认证项目是由《新英格兰医学杂志》和《NEJM医学前沿》联合制作,J-Med承办运营的NEJM全球首个培训认证项目,致力于培养有能力设计并实施高影响力临床试验的高水平临床研究人员。培训为期半年,线上线下形式相结合。
培训项目着重互动式和小班化教学,利用研修班、专题讲座、辅导课、研讨会等多种课程模式,由NEJM主编团队领衔,召集30余位来自哈佛、约翰.霍普金斯等高校医学院及其教学医院,及曾领导大型临床试验的知名专家组成项目师资团队。每周课程之余,学员需完成小测验,期中期末考试,个人汇报,并在导师的指导下完成个人临床研究方案,最终获得来自《新英格兰医学杂志》和《NEJM医学前沿》颁发并认可的结业证书。
This 6-month hybrid live online, and in-person program is specifically designed for individuals who have prior experience with clinical trials and aspire to enhance their skills and knowledge to conduct more sophisticated and world-class trials. Our course focuses on moving beyond analytical epidemiology and statistics, allowing you to delve into the practical aspects of trial implementation while developing a more advanced understanding of trial design and data interpretation.
This program is designed and implemented by the New England Journal of Medicine (NEJM) and NEJM医学前沿, and organized by J-Med in China. Upon successful completion of the program, participants receive a Certificate of Completion from NEJM and NEJM医学前沿.
Online Application
第四期 NEJM 高水平临床研究培训认证项目学费为 95,000 元人民币 。
申请截止日期:2025年10月9日
Structure
Highlights
• 30+ 中美顶级导师阵容30+ highly experienced educators & research leaders
• 集合中外NEJM论文主要作者Includes top experts who have published papers in NEJM
• 148高互动学时(96学时线下)148 total hours of instruction
• 小班化互动式精英教育Small-group interactive teaching mode
• 达到医学顶刊的严格标准Meet the rigorous standards of the highest impact medical journals
• 专业导师辅导临床研究方案Design, Complete and Present an RCT Proposal with faculty feedback
• NEJM和NEJM医学前沿联合颁发认证证书Upon successful completion, participants receive a certificate from NEJM and NEJM医学前沿
Faculty



























部分讲师名单,持续更新中
Framework
Phase I, Phase II, Phase III Clinical Trials Theory and Design (Parallel, crossover designs)
• 优效性、等效性和非劣性试验Superiority, Equivalence, and Non-inferiority Trials
• 适应性临床试验Adaptive Designs
• 如何开展高影响力高质量的临床试验How to Run a High Impact Trial
• 试验结果的解读和发表Communication of Trial Results
• NEJM中外作者成功经验分享NEJM Authors Successful Experience Sharing
• 中外临床试验的监管The Regulation of Clinical Trials in China and Abroad
• 全球健康背景下的临床研究Clinical Research in a Global Health Context
Objectives
Demonstrate a strong understanding of fundamental clinical research concepts related to clinical trials.
Demonstrate an expert understanding of high impact clinical trial design.
Become facile at interpretation and presentation of clinical trial data.
Understand how to design, conduct and write a high impact trial suitable for submission to high-impact journals such as the New England Journal of Medicine.
Understand how to avoid common clinical trial design and implementation pitfalls.
Arrangement
开班时间:2025年10月
学制长度:6个月
学员人数:约90人(滚动录取)
授课语言:Core track英文,Professional Development Track中文或英文
授课地点:武汉
教学系统:Canvas平台




How to Apply
This program is designed for
• 希望开展高影响力研究的中年资临床试验人员Mid-career clinical trialist who aims to produce high impact work
Early-career clinical researcher with aptitude for clinical trial implementation
Fee
第四期 NEJM 高水平临床研究培训认证项目学费为95,000 元人民币。
所有培训认证项目课程的听课费、讲义费、学习资源、辅导资源的使用费用。学员参加线下课程模块的交通,住宿,用餐费用自理。
•方法1 银行账户汇款
•方法 2 J-Med 学习平台(小鹅通)移动端支付
可开具增值税专用发票,学费需在2025年10月16日之前完成支付。